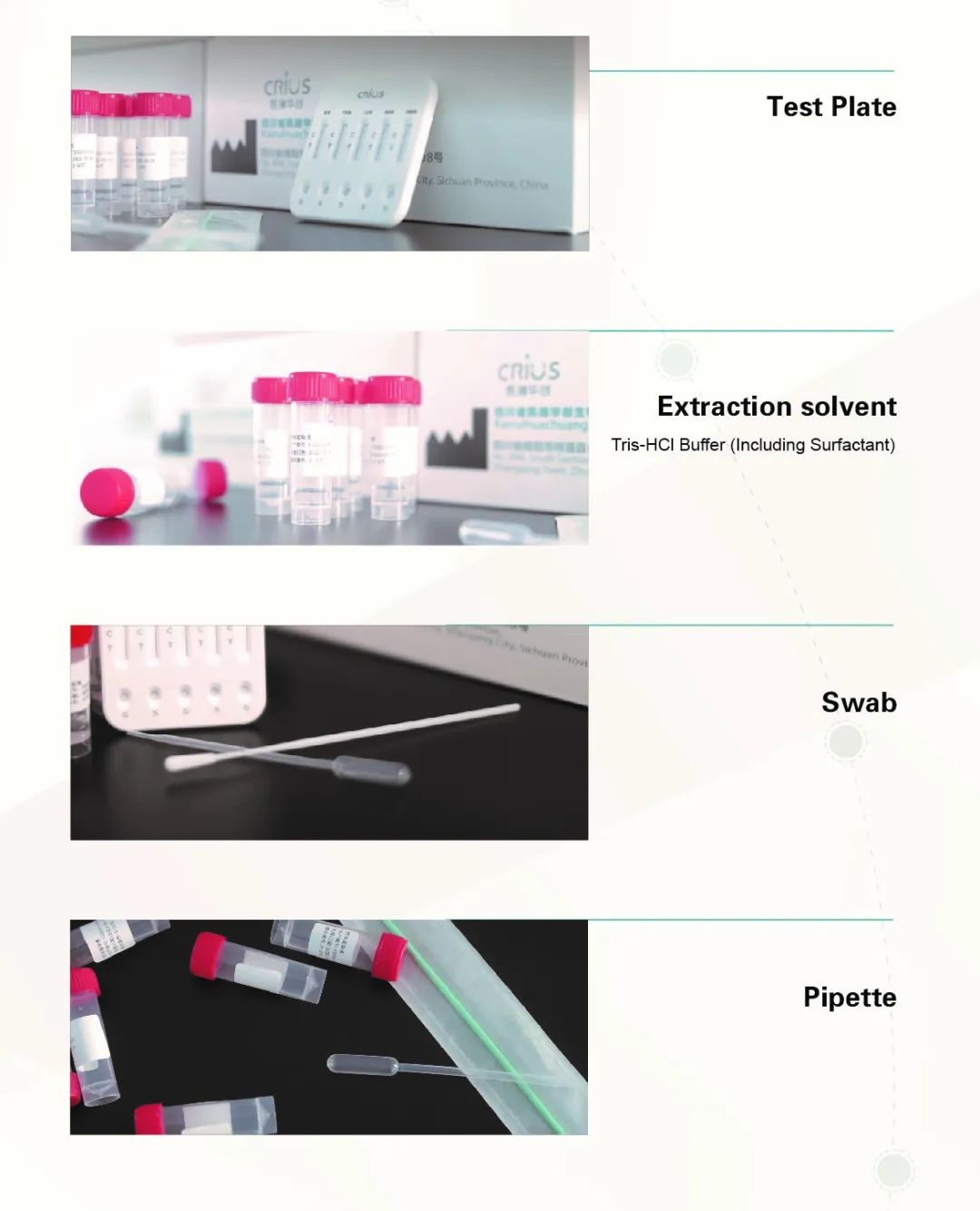
Influenza A Virus, Influenza B Virus, Respiratory Syncytial Virus,Respiratory Adenovirus
5-AntigenCombination Assay Kit
(colloidal gold method)
ProductIntroduction
The 5-Antigen Combination Assay Kit(colloidal gold method) produced by Sichuan CRIUS Biotech Co., Inc is mainly used for the detection of the antigens of novel coronavirus, influenza A virus,influenza B virus, respiratory adenovirus and respiratory syncytial virus inhuman nasopharyngeal and oral swab samples.

Since the beginning of the COVID - 19epidemic, both the viral nucleic acid detection and antibody detection havebeen widely used in China. However, most carriers of the virus could not beidentified in the early stages of infection. The primary causes are that the antibodydetection cannot provide accurate test results in the early stage due to itslow detection rate in the first two weeks of infection, and the viral nucleicacid testing is an extremely labor-intensive and time-consuming detectionmethod.In addition, thenucleic acid testing can only be performed in a professional laboratory becauseof its special requirements for testing equipment, environment and testingpersonnel.

Comparison of various detection methods fornovel coronavirus
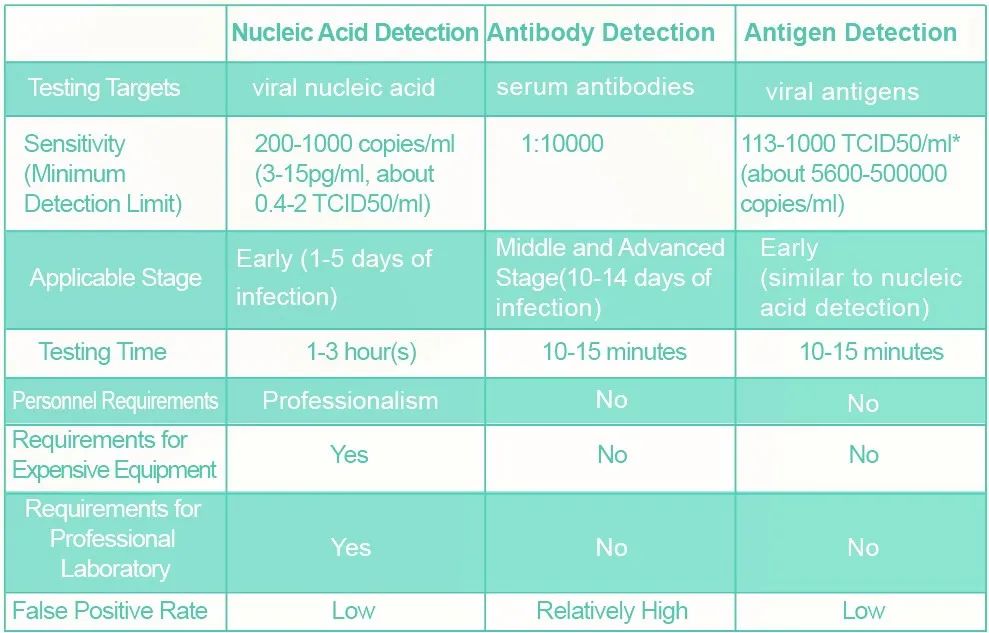
Note: * 1TCID50 contains 500 copies ofvirus. Data are obtained from the 3 reagents specification approved forclinical use in the Novel Coronavirus antigen assayLike RNA, structural proteins are specificmarkers of coronaviruses and are also viral antigens that stimulate theproduction of specific antibodies in patients. As shown in the above table,viral antigen detection has not only the advantages of antibody detection(rapid, simple, convenient, economical and efficient), but also of nucleic aciddetection, including applying to early diagnosis and high reliability.
It is worth noting that, in the coronavirusparticles, the antigen content can reach thousands of times more than that ofnucleic acid. A coronavirus particle contains only one RNA, while theaverage content of S1 protein and N protein is 222 and over 3000 copies,respectively. In light of that, when the minimum detection limit of S1antigen reaches 6-30pg/mL or N antigen reaches 40-200pg/mL, its sensitivitycould be equal to that of nucleic acid detection (200-1000 copies /ml, i.e.,3-15pg/ml) . Therefore, antigens are more ideal detection targets thannucleic acids theoretically. If permitted, the rate of missed detectioncould be greatly reduced with the two methods being used simultaneously.

Value of Combination Assay Kit inAnti-epidemic
According to the Scheme for Diagnosis andTreatment of 2019 Novel Coronavirus Pneumonia (The 8th Trial Edition) issued bythe National Health Commission in August 2020, COVID-19 should be mainly distinguishedfrom the known viral pneumonia caused by influenza virus, adenovirus,respiratory syncytial virus and from mycoplasma pneumoniae infection. Inparticular, for suspected cases, testing methods including rapid antigendetection and multiplex PCR nucleic acid detection should be adopted as far aspossible so as to identify common respiratory pathogens.The development of CRIUS 5-AntigenCombination Assay Kit fully meets the requirements of the national guidelinesand has great clinical significance in the diagnosis and treatment of COVID-19.When there are suspected symptoms, initial self-test and infectionmanagement can be carried out at home (home self-test is subject to localregulations), which can quickly predict whether it is COVID-19 or an infectioncaused by influenza or other viruses, and thus plays a crucial role in theprevention and control of COVID-19. Especially for the elderly, infants andother susceptible population, it is helpful for rapid identification, effectivetreatment and prevention of cross-infection.